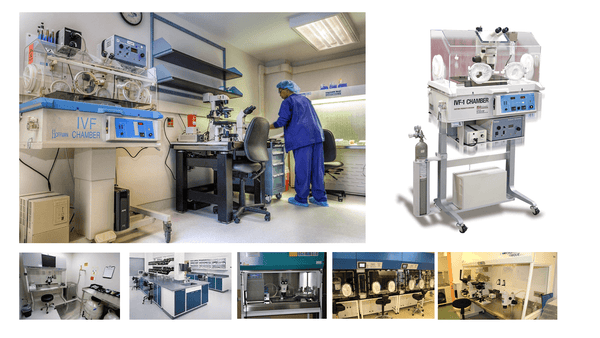
What we know and do not know about requirements for lab design

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
What we know and do not know about requirements for lab design
GILES PALMER

Giles Palmer is a senior clinical embryologist, skilled in Laboratory, Business and Quality management.
After graduating in Genetics at Leeds University, UK he attained a position as research officer at London's Hammersmith Hospital's acclaimed IVF unit working with Professors Lord Winston and Alan Handyside.
After a consultancy position for Iceland's first IVF Unit (University Hospital, Reykjavik) he moved to Greece in 1992.
Since moving to Greece, he has worked in the largest IVF units in Athens.
He was Director of the highly successful Assisted Reproduction Unit at Mitera hospital in Athens 2002-2016.
His collaboration with St. Sophia's Children's Hospital (Athens University) resulted in the first birth in Greece following embryo-biopsy and pre-implantation genetic diagnosis, and has led to publications in Leading Scientific journals concerning many topics including Pre-implantation Genetic Diagnosis for Cystic Fibrosis and B-thalassaemia.
He is a member of ESHRE (European Society of Human Reproduction and Embryology), ARCS (The Association of Reproductive and Clinical Scientists), PEKE (Greek Society of Clinical Embryologists) , SLTB (Society of Low Temperature Biology) and has been accredited with Senior Embryologist Status by ESHRE.
More recently he has become a consultant and product developer in a wide range of areas within the industry including cleanroom technology; quality management; risk assessment for clinics & cryo-storage facilities and Artificial Intelligence in ART.