Quality Control in the Cloud

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
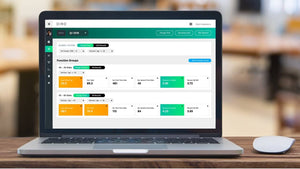
IVF Quality Control in the Cloud
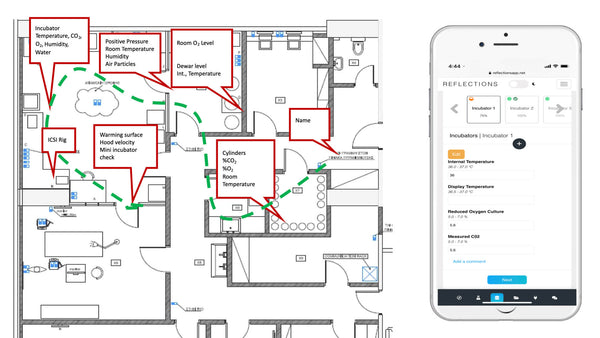
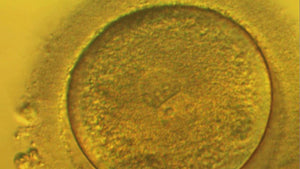
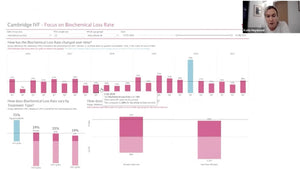
Periodic laboratory monitoring is essential for timely correction of differences between desired and measured parameters such as temperature. Despite this well-accepted principle, little is known of periodic recording practice. Usually records are filed away and used only rarely on audits and inspections
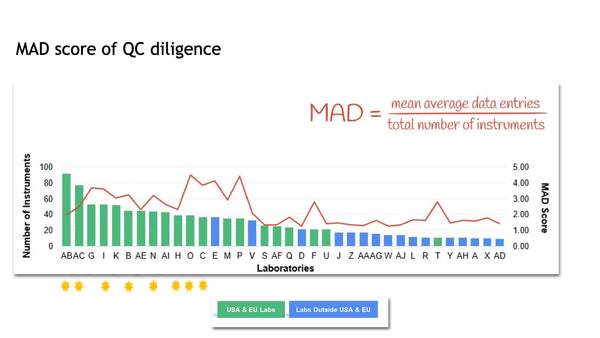
In the IVF industry, unlike other industries, cloud computing has not been widely used in quality control and little evidence exists that clinics reflect upon the results. This presentation highlights the different practises of QC in the lab around the world, describes a recent publication that looked at QC habits globally in the lab and proposes the ”MAD score” for laboratory quality control diligence.