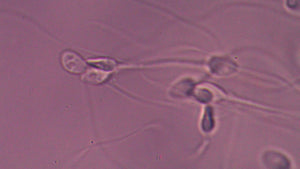
Sperm on Drugs

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
Sperm on Drugs
DR DAVID MORTIMER

Originally from the UK: BSc (Hons) Zoology from Bristol (UK) 1974 and PhD (Edinburgh) 1977; post-doctoral fellowships in Edinburgh, Paris and Birmingham; Faculty member at the University of Calgary (Canada) in Obs & Gynae and Medical Physiology 1983–1991. Research focussed on human sperm pathophysiology. Scientific Director of Sydney IVF (Australia) 1991–1999. Co-founder of the ESHRE Basic Semen Analysis courses in 1994. President of the Canadian Fertility and Andrology Society 2009–2010; Associate Editor of Human Reproduction Update 2011–2014. Member of Accreditation Canada’s ART Standards Working Group since 2007, and of the Canadian Standards Association since 2008. Honorary Senior Lecturer, University of Dundee School of Medicine (UK) since 2017.
David has given many invited lectures and taught in workshops and courses worldwide. Author/co-author of almost 300 conference presentations and 160 publications, including the books Practical Laboratory Andrology (Oxford University Press, 1994), Quality and Risk Management in the IVF Laboratory (Cambridge University Press, 2005; 2nd edition 2015), and A Practical Guide to Basic Laboratory Andrology (Cambridge University Press, 2010). Academic publications h-index = 39.
He became a full-time consultant in 1999 and, with his wife Dr Sharon Mortimer, established the Vancouver-based international reproductive biomedicine consulting company, Oozoa Biomedical, in 2000. Major activities include designing new ART laboratories and cryobanks, upgrading or implementing new lab systems, helping laboratories develop Total Quality Management programmes, performing TQM audits, and advising companies developing products for the reproductive biomedicine field.
He currently serves as Scientific Advisor to Mellowood Medical (Canada), Planer (UK), Hamilton Thorne (USA) as well as several ART centres internationally.