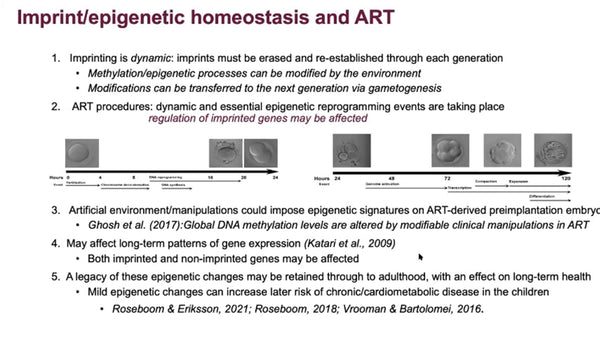
The Embryo in Culture: Imprinting, Oxidative Stress and Epigenetic Homeostasis

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
Session 69: The Embryo in Culture: Imprinting, Oxidative Stress and Epigenetic Homeostasis
Tuesday 22nd June, 2021. 3PM EST/ 8PM GMT / 9PM CET
Sponsored by Cook Medical
DR. KAY ELDER
 Following an academic career that included degrees in biochemistry (BSc, University of St Andrews), molecular biology (PhD, University of Colorado Medical School) and medicine (MBBChir, Cambridge), Kay Elder joined the Bourn Hall team as Clinical Assistant to Patrick Steptoe in 1984, directing the Out-Patient Department from 1985-1987 before joining the IVF lab as Senior Embryologist. Prior to medical studies in Cambridge, she was a research scientist at Imperial Cancer Research Fund in London. In 1989 she initiated and directed a program of Continuing Education for IVF doctors, scientists and nurses for the next 16 years, during which time she helped to set up and run two Master’s degree programs in Clinical Embryology. Kay was appointed Deputy Editor to Bob Edwards for the journal RBMonline from 2005. She has published 8 textbooks for IVF students and continues to mentor and tutor postgraduate students.
Following an academic career that included degrees in biochemistry (BSc, University of St Andrews), molecular biology (PhD, University of Colorado Medical School) and medicine (MBBChir, Cambridge), Kay Elder joined the Bourn Hall team as Clinical Assistant to Patrick Steptoe in 1984, directing the Out-Patient Department from 1985-1987 before joining the IVF lab as Senior Embryologist. Prior to medical studies in Cambridge, she was a research scientist at Imperial Cancer Research Fund in London. In 1989 she initiated and directed a program of Continuing Education for IVF doctors, scientists and nurses for the next 16 years, during which time she helped to set up and run two Master’s degree programs in Clinical Embryology. Kay was appointed Deputy Editor to Bob Edwards for the journal RBMonline from 2005. She has published 8 textbooks for IVF students and continues to mentor and tutor postgraduate students. In her current role as Senior Research Scientist at Bourn Hall, she co-ordinates research collaborations with academic research establishments and provides information and counselling for patients who wish to donate gametes or embryos to research.
PROFESSOR YVES MENEZO
REFERENCES
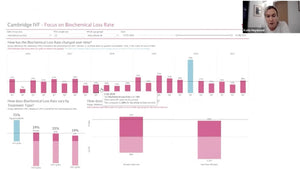
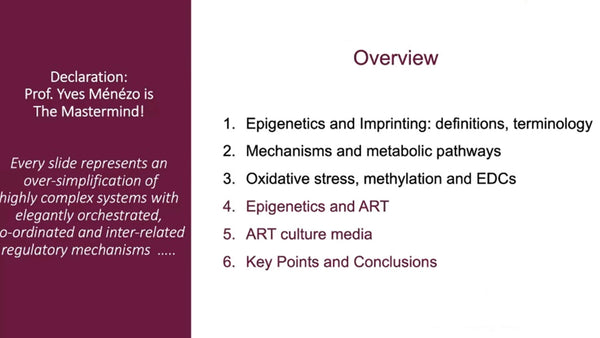
Epigenetic homeostasis and human embryo culture
Kay Elder International IVF Initiative, June 22nd 2021
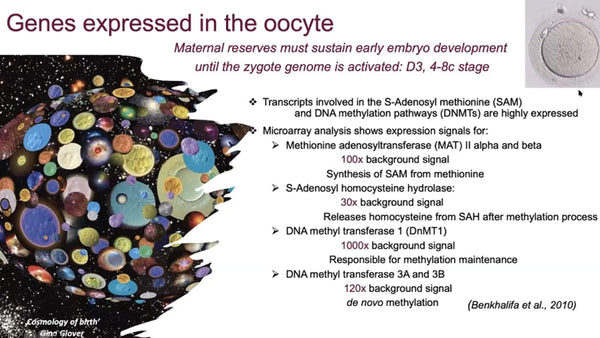
Benkhalifa M, Montjean D, Choen-Bacrie D, Ménézo Y (2010) Imprinting: RNA expression for homocysteine recycling in the human oocyte. Fertility & Sterility 93(5): 1585-1590.
Boxmeer JC, Smit M, Utomo E et al. (2009) IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum Reprod. 24:1059-66.
Butler, MG (2009) Genomic imprinting disorders in humans: a mini-review. JARG 26:477-486.
Carli D, Riberi E, Battista Ferrero G, Mussa A (2020) Syndromic disorders caused by disturbed human imprinting. J Clin Res Pediatr Endocrinol 12(1): 1-16
Cao J, Wu Q, Huang Y et al. (2021) The role of DNA methylation in syndromic and non-syndromic congenital heart disease. Clin Epigenetics 13:93.
Chang AS, Moley KH, Wangler MS et al. (2005) Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: A case series of 19 patients. Fertility & Sterilty 83(2): 349-354.
Chen Z, Robbins KM, Wells KD, Rivera RM (2013) Large offspring syndrome: a bovine model for the human loss-of-imprinting overgrowth syndrome Beckwith Wiedemann. Epigenetics 8(6):591-601.
Cooney, C (1999) Methyl Magic: Maximum Health Through Methylation. Angus McMeet, Pub.
Fauque P, De Mouzon J, Devaux A et al. (2020).Reproductive technologies, female infertility, and the risk of imprinting-related disorders. Clinical Epigenetics 12:191
Ferguson-Smith AC (2011) Genomic imprinting: the emergence of an epigenetic paradigm. Nature Reviews (Genetics) 12: 565-575.
Fowler B (2005) Homocysteine: overview of biochemistry, molecular biology, and role in disease processes. Semin Vasc Med. 5(2):77-86.
Giorgioni V, Parazzini F, Fesslova V et al. (2018) Congenital heart defects in IVF/ICSI pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol 51: 33-42.
Ghosh J, Coutifaris C, Sapienza C, Mainigi M (2017) Global DNA methylation levels are altered by modifiable clinical manipulations in assisted reproductive technologies. Clinical Epigenetics 9:14 (eCollection doi: 10.1186/s13148-017-0318-6)
Hatori H, Hiura H, Kitamura A et al (2019) Association of four imprinting disorders and ART. Clinical Epigenetics 11(1): 21. doi: 10.1186/s13148-019-0623-3.
Hiura H, Okae H, Naoko M et al. (2012) Characterization of DNA methylation errors in patients with imprinting disorders conceived by assisted reproduction technologies. Human Reproduction 27(8): 2541-2548.
Huffman, S.R., Pak, Y., & Rivera, R.M. (2015). Superovulation induces alterations in the epigenome of zygotes, and results in differences in gene expression at the blastocyst stage in mice. Molecular Reproduction and Development 82: 207–217.
Huntriss, J (2020) Epigenetics and human assisted reproduction. In: In-Vitro Fertilization, 4th ed., Ch 15. K Elder & B Dale, Cambridge University Press.
Katari S, Turan N, Bibikova M et al. (2009) DNA methylation and gene expression difference in children conceived in vitro or in vivo. Human Molecular Genetics 18(20): 3769-3778.
Lazaraviciute G, Kauser M, Bhattacharya S et al (2014) A systematic review and meta-analysis of DNA methylation levels and imprinting disorders in children conceived by IVF/ICSI compared with children conceived spontaneously. Human Reproduction Update 20(6): 840-852.
Maher ER (2003) Imprinting and assisted reproductive technology. Human Molecular Genetics 14(1): R133-R138
Market Velker BA, Denomme MM, Mann MR (2012). Loss of genomic imprinting in mouse embryos with fast rates of preimplantation development in culture. Biol Reprod. 86(5):143, 1-16.
Market-Velker BA, Fernandes AD, Mann MR (2010). Side-by-side comparison of five commercial media systems in a mouse model: suboptimal in vitro culture interferes with imprint maintenance. Biol Reprod. 83:938-50.
Ménézo Y, Clement P, Clement A, Elder K (2020). Methylation: an ineluctable biochemical and physiological process essential to the transmission of life (Review). International Journal of Molecular Sciences 21, 9311 https://doi.org/10.3390/ijms21239311.
Ménézo Y, Clement P, Dale B, Elder K (2021) Modulating oxidative stress and epigenetic homeostasis in preimplantation IVF embryos. Zygote, in press.
Ménézo Y, Clement P, Dale B (2019) DNA methylation patterns in the early human embryo and the epigenetic/imprinting problems: a plea for a more careful approach to human Assisted Reproductive Technology (ART). Int J Mol Sci 20(6):1342. https://doi.org/10.3390/ijms20061342.
Ménézo Y, Dale B, Elder K (2017). Time to re-evaluate ART protocols in the light of advances in knowledge about methylation and epigenetics: An Opinion paper.
Human Fertility 21(3): 1-7.
Ménézo Y, Elder K (2020) Epigenetic remodelling of chromatin in human ART: addressing deficiencies in culture media (Review) JARG 37: 1781-1788.
Ménézo Y, Elder K, Benkhalifa M, Dale B (2010) DNA methylation and gene expression in IVF. RBM Online 20 (6): 709-710.
Ménézo Y, Khatchadourian Ch, Garib A et al. (1989) Regulation of S-adenosyl methionine synthesis in the mouse embryo. Life Sciences 44: 1601-1609.
Ménézo Y, Lichtblau I, Elder K (2013) New insights into human pre-implantation metabolism in vivo and in vitro. JARG 30(3):293-303.
Ménézo Y, Elder K, Dale B (2019) The negative impact of the environment on methylation/epigenetic marking in gametes and embryos: A plea for action
to protect the fertility of future generations. Molecular Reproduction and Development 86(10):1273–1282.
Ménézo Y, Elder K, Dale B (2015). A link between increased prevalence of ASD syndromes and oxidative stress, DNA methylation and imprinting: the impact of the environment. JAMA Pediatrics 169(11). DOI 10.1001/jamapediatrics.2015.2125.
Ménézo Y, Mares P, Cohen M et al (2011). Autism, imprinting and epigenetic disorders: a metabolic syndrome linked to anomalies in homocysteine recycling starting in early life?? JARG 28(12): 1143-5.
Ménézo Y, Silvestris E, Dale B, Elder K (2016) Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reproductive Biomedicine Online 33 (6): 668-683.
Morbeck DE, Krisher RL, Herrick JR et al. (2014) Composistion of commercial culture media used for human embryo culture. Fertility & Sterility 102: 759-766
Reik W, Walter J (2001) Genomic imprinting: parental influence on the genome.
Nature Reviews (Genetics) 2(1): 21-32
Roseboom TJ & Erikson JG (2021) Children conceived by ART grow differently in early life than naturally conceived children but reach the same height and weight by age 17. Reassuring? Not so sure. Human Reproduction 36(4): 847-849.
Roseboom TJ (2018) Developmental plasticity and its relevance to assisted human reproduction. Human Reproduction 33(4):546-552.
Sharpe RM (2018) Of mice and men: long-term safety of assisted reproduction treatments. Human Reproduction 33 (5): 793–796.
Soda K (2018) Polyamine metabolism and gene methylation in conjunction with one-carbon metabolism. Int J Mol Sci 19(10):3106.
Song S, Ghosh J, Mainigi M et al. (2015) DNA methylation differences between in vitro- and in vivo-conceived children are associated with ART procedures rather than infertility. Clinical Epigenetics 7: 41.
Stuppia L, Franzago M, Ballerini P et al (2015) Epigenetics and male reproduction: the consequences of paternal lifestyle on fertility, embryo development, and children lifetime health. Clinical Epigenetics 7: 120. doi: 10.1186/s13148-015-0155-4
Sunde A, Brison D, Dumoulin J et al (2016). Time to take human embryo culture seriously. Human Reproduction 31(10):2174-82.
Tisato V, Silva JA, Longo G et al. (2021) Genetics and epigenetics of one-carbon metabolism pathway in autism spectrum disorder: a sex-specific brain epigenome (Review). Genes 12: 782
Vrooman LA & Bartolomei MS (2017) Can assisted reproductive technologies cause adult-onset disease? Evidence from human and mouse. Reprod Toxicology 68: 72-84.