Session 105: Quality inside

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
Session 105: Quality inside
8th Nov 2022, 3pm EST LIVE, 8pm UK, 9pm CET
This Webinar is sponsored by the Reflections App from IVFqc
Course outline/synopsis:
Description: International IVF Initiative(I3)- Online Session 105: QUALITY INSIDE
Tuesday, 8th Nov 2022, 3pm EST LIVE, 8pm UK, 9pm CET [ 109mins]
Moderators:
Dr. Jean Popwell
Cassie Miller
Presenters:
Dr. Mina Alikani “Reflections on quality control”
Dr. Thomas B. (Rusty) Pool “QC goes to the cloud, comes back QI”
Dr. Gerardo Mendizaba Ruiz "The internet of IVF things"
Michael Skumial "Operational software in IVF and staff scheduling”
With Dr. Alison Campbell and Ineabelle Collazo ( Online version only)
Q and A
Moderated by Senior Embryologists Dr. Jean Popwell and Cassie Miller with years of experience in quality management this webinar featured current quality measures that are evident in IVF clinics across the globe and best practise- highlighting new technologies to help embryologists to obtain a robust quality control (QC) system for lab equipment and environment.
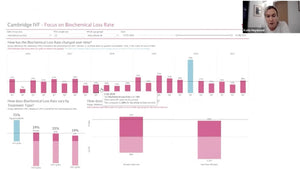
Dr Mina Alikani, President of Alpha Scientists in Reproductive Medicine discussed current shortcomings IVF lab QC and talked about cloud-based systems that can aid lab managers in data collection, data monitoring (onsite and remotely), analysing fluctuations and reporting.
This was followed by Dr. Thomas B. (Rusty) Pool who gave examples of QC practises in other industries where paper forms and clipboards are replaced by tablets and phones that transcribe and record simultaneously. Dr Pool returned to the IVF lab to show how with real life examples how electronic recording can trouble shoot in the laboratory.
Dr. Gerardo Mendizaba Ruiz, an electronic engineer, spoke of networks of connecting devices using fog/edge computing forming a concept called the “Internet of Things”. With examples already used in homes and in healthcare, this lecture explained how a network of devices controlled through the internet and communicating with each other could be a great advancement in the IVF lab with increased data collection, improved traceability and more efficient use of protocols through machine learning.
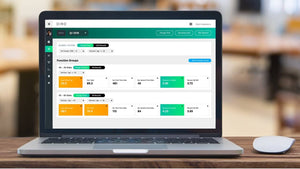
Finally, Michael Skumial discussed “Operational software in IVF” and highlighted better methods of organising the routine administrative and quality duties that are essential in a clinics day to day running with emphasis on staff scheduling and how complex programs and interconnecting departments could use bespoke software to better prepare for the workload in an IVF clinic any day of the year.
The session concluded with an extensive Questions and Answers section that invited questions from the audience to participate which including all presenters and moderators discussing Quality Management and technology.
DR. JEAN POPWELL
An interest in expanding her experience and interests propagated involvement in other opportunities as a biopsy trainer and lecturer for Natera, Irvine Scientific and Illumina, helping embryologist learn Day 5 culture systems and trophectoderm biopsy across the US and other countries. Jean also worked as an off-site Laboratory Director for 2 years and provided IVF lab consulting to other laboratories regarding their overall quality management systems and procedures.
Jean strives to remain involved in both local, Northern California Association of Reproductive Biologists and national, College of Reproductive Biologists of the Association of American Bioanalysts, organizations that support embryologist educational and professional needs and has provided support as Vice President, President, and Past President in these organizations. Currently, she is Vice President of the Southwest Embryology Summit (SWES).
In 2021, Jean Popwell, became the Laboratory QA/QC Director at Inception Fertility and strives to maintain and support laboratory directors in their efforts toward successful patient outcomes for their clinics.
CASSIE MILLER
Cassie Miller, Ovation® Fertility’s director of risk management, helps Ovation’s IVF labs maintain regulatory compliance while creating opportunities for next-level information and data sharing to drive performance improvement and reduce risk across the organization.
Miller’s primary focus is ensuring that Ovation’s employees have what they need to work safely, that embryos are protected, and that patient identification and privacy are maintained. By gathering, analyzing and sharing organization wide quality management data, she proactively identifies risks and implements appropriate preventative actions to minimize, mitigate or eliminate issues before they occur.
Miller joined Ovation in July 2019 as compliance manager, ensuring that each of Ovation’s laboratories were in compliance with all rules, regulations and laws that relate to the fertility care field. In this role, she standardized Ovation’s regulatory response and helped Ovation labs address ever-changing standards for privacy and safety, helping to ensure delivery of high-quality patient care.
Before joining Ovation, Miller served as Midwest territory manager for Irvine Scientific. She also has more than 20 years of hands-on clinical laboratory experience, including three years as an embryologist, andrologist, endocrinology specialist, and interim supervisor for Bennett Fertility Institute in Oklahoma City.
Earlier in her career, she spent more than a decade as a laboratory supervisor, lead medical technologist and blood bank supervisor for OU Medicine, Inc., in Edmond, Oklahoma. Prior to that, she worked for nearly nine years as a lead medical technologist for AllianceHealth Deaconess in Oklahoma City.
DR. THOMAS B (RUSTY) POOL
DR. GERARDO MENDIZABA RUIZ
MICHAEL SKUMIAL
Proficient with operations and technology within various healthcare domains including operations, finance, clinical, surgical, research, education, private practice, laboratory, human resources, legal, administrative, regulatory and compliance, revenue, social media, online presence, medical electronic records and mergers and acquisitions.
Fertility Connect is an IVF operations management software suite developed by Health Connect Algorithms. https://healthconnectalgorithms.com/
DR. MINA ALIKANI
Mina has lectured worldwide on ART topics and has authored and co-authored more than 80 articles and book chapters on laboratory aspects of assisted reproduction. She is an editor of RBMO and currently the President of Alpha Scientists in Reproductive Medicine. She also serves on the Scientific Advisory Board of TMRW Life Sciences.
DR. ALISON CAMPBELL
Dr Alison Campbell is the Chief Scientific Officer at CARE Fertility, an expanding and leading clinical group consisting of 14 embryology laboratories and around 150 Scientists, in the UK and Ireland. Alison has over 25 years experience in the field in Reproductive Medicine and has played a key role in establishing and integrating new laboratories, driving standards and best practice, and leading scientific research and development across the CARE group. Alison is a Fellow of the Royal College of Pathologists, an Associate Editor of Human Reproduction, a Section Editor of RBM Online and a member of the Alpha Scientists in Assisted Reproduction Executive Board. Alison has recently established a Masters degree course in clinical embryology in partnership with Liverpool John Moore’s University Masters degree in Clinical Embryology.
INEABELLE COLLAZO
Ineabelle is the Embryology Laboratory Director at IVFMD in Florida. She currently manages 4 IVF labs and 6 Andrology labs in the South Florida region. Her role at IVFMD has been critical into bringing technology in the laboratory as a way to standardize, improve outcomes and running an effective and efficient laboratory. Ineabelle has a vast experience in management of an embryology laboratory that is backed up by over 30 years in the field. She has co-authored several abstracts and has been a guest speaker at several summits. Her main objective is to integrate laboratory personnel in technology innovations as a way to provide the best quality of care possible