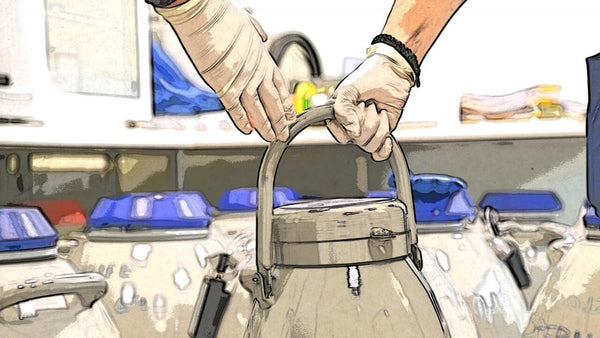
Session 103: CRYOGOVERNANCE V - BEST PRACTICES IN CRYOTRANSPORTATION

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
Session 103: CRYOGOVERNANCE V - BEST PRACTICES IN CRYOTRANSPORTATION
Description: International IVF Initiative(I3)- Online Session 103-CRYOGOVERNANCE V - BEST PRACTICES IN CRYOTRANSPORTATION
Tuesday, Tuesday, 11th October at 3pm EST, 2022. [105 mins]
Course outline/synopsis:
Moderated by Senior Embryologists Giles Palmer (UK) and Jessica Bailey (USA), this online webinar dealt with issues concerning shipping gametes and embryos.
The increased laboratory workload together with the administration required for safe shipment of samples has become an increased burden that is faced by clinical embryologists who are required to transport samples from clinic to clinic, both nationally and globally. Patients’ own biological samples and donor oocytes and sperm are requiring specific new ISO standards and in the wake of the pandemic the transport lines are still at reduced capacity.
Several aspects of current working conditions, shortfalls and specific requirements that are enforced by clinics are discussed: Dr. Kristen Ivani (USA), consultant embryologists, described good practise and examples of protocols to ensure safe shipment from an embryologists point of view while Deborah Mecerod, Director of Clinical Operations for a US egg bank , presented her experiences and special needs required to manage and ship donor eggs from a large national egg bank.
Bret Bollinger (USA), Chief Technical Officer, for Cryoport Systems, in a recording presentation, described the latest technology in live tracking samples in transit with unique parameters such as impact and tilt functions and showcased a new type of spherical dry shipper that stays always upright. Also, more efficient ways to manage the fleet of dry shippers with validation and paperless transportation was presented.
Finally, Dr. Mark Sawicki (USA) discussed the future of cry transportation including the increasing demands for validation and auditing of transport services in the light of both the ISO standard ISO 21973 and recent regional regulations such as the Fertility Society of Australia New Zealand (FSANZ) – Technical bulletin 13 – “Transport of Cryopreserved Gametes and Embryos”
The webinar was concluded by a question and answer section including all of the speakers plus Victoria A. Wells, ( senior embryologist UK) and John Condon (USA) with Dr. Simon Cooke (Scientific director of IVF Australia) joining live from Australia explaining his experience and demands for his network of clinics in relation to precious cargo shipping.
This session is sponsored by Cryoport
Moderators:
Giles Palmer
Jessica Bailey
Panelists:
Victoria A. Wells, John Condon and Dr. Simon Cooke
Presenters:
Dr. Kristen Ivani : The embryologist's point of view
Deborah Mecerod: Demands of an egg bank
Bret Bollinger: The role of technology in IVF shipping
Dr. Mark Sawicki: The future of cryotransportation
Q and A
DR. KRISTEN IVANI
Kristen Ivani completed both her BS and MS degrees in Animal Science with an emphasis in reproductive physiology. She earned her PhD degree in Physiology with the Animal Reproduction and Biotechnology group at Colorado State University. Kristen served as the IVF Laboratory Director at the Reproductive Science Center of the San Francisco Bay Area in San Ramon, CA for over 30 years before recently turning over the reins and being promoted to part-time embryologist and consultant.
In her free time, she enjoys sharing her love of embryology, studying the legal and ethical aspects of ART, and doing anything outdoors. She also enjoys her “other” director job as a volunteer camp director at Two Sentinels Girl Scout camp which has provided backpacking experiences in the Sierra Nevada mountains for over 80 years.
DEBORAH MECEROD
Deborah Mecerod, Clinical Nurse Manager, joined Reproductive Biology Associates in 1998. A native of Long Island, N.Y., she graduated from Catholic Medical Center School of Nursing and attended Nassau Community College.Deborah has over 30 years of experience in the field of Infertility. Her career started at North Shore University Hospital, Manhasset, N.Y. in 1988 as an IVF nurse. She relocated to Atlanta, Georgia, in 1996, and has worked at RBA for 19 years. Deborah served as the Clinical Nurse Manager for 13 years. In July of 2011, Deborah was appointed Director of Clinical Operations for MyEggBank. Notably this was the first U.S egg bank for frozen donor eggs.
Most recently Deborah moderated and spoke at the 2018 ARSM scientific Congress “There’s No Such Thing as Anonymous”. Deborah has co-chaired the 2009 and 2010 “International Nursing Conference on Reproductive Health and Infertility”. Deborah served as an active chair for the 2011 “Start Art Conference” in Las Vegas. Deborah has spoken at several nursing conferences about egg donation, vitrification and FDA guidelines.
Deborah enjoys spending time with her 3 children, family and friends.
In her spare time Deborah enjoys traveling to new destinations; cooking; kickboxing and weight lifting.
BRET BOLLINGER
DR. MARK SAWICKI
Mark Sawicki brings 15 years of business development and sales management experience, having consistently delivered on corporate revenue and market share goals in the pharmaceutical and biotechnology industries. Sawicki was most recently the chief business officer at AAIPharma Services Corporation/Cambridge Major Laboratories Inc. Additionally, he has served in senior business development roles at CMC Biologics and Albany Molecular Research Inc. (AMRI), where he increased revenue at rates far outpacing industry standards. Sawicki holds a bachelor’s in biochemistry from the State University of New York at Buffalo and a Ph.D. in biochemistry from the State University of New York at Buffalo, School of Medicine and Biomedical Sciences. He also received graduate training at the Hauptman Woodard Medical Research Institute. Sawicki has authored a dozen scientific publications in drug discovery with a focus on oncology and immunology.
GILES PALMER
He is the executive director for this non profit global educational project in Assisted Reproductive Technologies (ART), the International IVF Initiative.
JESSICA BAILEY
DR. SIMON COOKE
Working in and overseeing the management of embryo and sperm laboratories since 1992, Simon has driven multiple laboratory quality and automation projects over the last 30 years within the labs, aimed at improving quality and safety, and reducing the time to pregnancy.
JOHN CONDON
John’s experience in the IVF/Reproductive Medicine spans over 30 years, previously representing CooperSurgical Fertility and Genomic Solutions, Smiths Medical – Wallace and Irvine Scientific in the consumable, equipment, and genomic solutions business. John holds a BA degree from The University of Massachusetts at Amherst in Health Administration and is currently resides in Rhode Island.
VICTORIA A. WELLS
Victoria graduated from Sheffield University in 2000 with a BSc in Biochemistry and gained her ACE certificate in Embryology in 2005, followed by HCPC registration in 2007. She worked in both the NHS and private sectors, in both locum and permanent positions; her most recent London based job was as a team-leading Senior Embryologist at the Lister fertility clinic, one of the largest clinics in the UK.
Her particular areas of interest include male factor subfertility and she sat on an MDT panel as the representative Embryologist in a regular discussion group which included international Urologists and Andrologists.
An accomplished ICSI practitioner of over 15 years and Embryo Biopsy Practitioner of over 10 years Victoria has recently joined the Agora Clinic in Sussex where she is leading the implementation of a PGT programme.
.