Session 132: The Night Watch

Donate
At the International IVF Initiative, we are committed to providing free access to our educational sessions, webinars, and resources for professionals and individuals passionate about advancing reproductive medicine. We believe that cost should never be a barrier to knowledge and collaboration. By contributing, you’re ensuring that valuable educational resources, expert insights, and collaborative opportunities remain open to all without financial barriers. Together, we can continue to foster a global community dedicated to innovation and excellence in the field of IVF.
Your Donation
Thank you!
Session 132: The Night Watch
3pm ET/ 8pm UK/ 9pm CET, Tuesday 14th May 2024.
Synopsis:
During the webinar, 120 minutes, moderated by Embryologists Dr. Jacques Cohen, Dr. Carol Loscher, and Ian Muusha, several experts presented insightful talks.
First, Matt Guy, a Chemical Engineer & Biotechnologist, discussed "Predictive maintenance of IVF equipment through IoT." He highlighted the application of current technology in predictive maintenance for IVF equipment
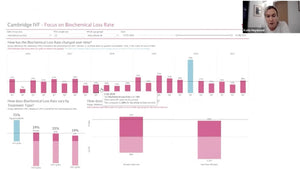
Following this, Dr. Neelke De Munck, head of a large IVF clinic, addressed "Equipment during day and night." She focused on real-time monitoring and data entry via daily lab logs performed by embryologists, offering valuable perspectives
Lastly, Dr. Michael G. Collins shared his research on data chain of custody in cryostorage, embryologist fatigue, and advancements in monitoring liquid nitrogen vessels in his talk, "The impact of continuous data collection and analysis." He emphasized the critical importance of data review.
The session concluded with an engaging Q&A session, allowing attendees to delve deeper into the discussed topics and address any lingering questions.
Moderators:
Dr. Jacques Cohen, Dr. Carol Loscher, Ian Muusha
Presenters:
Matt Guy: Predictive maintenance of IVF equipment through IoT
Dr Neelke De Munck: Equipment during day and night
A talk kindly sponsored by the Reflections App
Dr Michael G. Collins: The impact of continuous data collection and analysis
A talk kindly sponsored by TMRW Life Sciences
Q and A
REGISTER HERE
Dr. Jacques Cohen
Dr. Jacques Cohen is a reproductive biologist, laboratory product developer and high complexity laboratory director (HCLD). He is product developer and co-founder of IVFqc/Althea Science, which develops software solutions for laboratories and clinics. He was co-founder of Reprogenetics, a PGD service, now continuing as Cooper Genomics. He was a co-founder and product developer of Life-Global/IVF-Online. He has (co-)authored more than 300 publications, several textbooks and 12 patents. He is Emeritus Chief Editor of Reproductive Biomedicine Online.
He was one of the founders of Alpha – Scientists in Reproductive Medicine and one of the founders of Preimplantation Genetic Diagnosis International Society - PGDIS. He is a founding member of the International IVF Initiative.
Jacques Cohen was born in The Hague, The Netherlands and graduated in Biology of Reproductive Science in 1978 at Leiden University, Leiden, The Netherlands. He has a Ph.D. from Erasmus University in Rotterdam (supervisor Prof. Gerard Zeilmaker), in aspects of in vitro fertilization and male factor infertility. His postdoctoral studies (1982-1985) were performed at Cambridge University (UK) and Bourn Hall Clinic (supervisor Prof Robert Edwards). Jacques Cohen has (co-)developed a number of embryological methodologies and devices: blastocyst cryopreservation, assisted fertilization, assisted hatching, preimplantation genetic testing, ooplasmic donation, single sperm freezing, CODA filtration, Global media, and GPS dishes. He currently serves on Advisory Boards of biotech start-ups, TMRW, Kindbody and is CSO of IVF 2.0 Ltd., and Conceivable Life Sciences.
Dr. Carol Loscher
With a background in Pharmacology and a PhD in Genetics, Dr. Loscher entered the field of Embryology and has gathered a wealth of experience within Ireland’s leading fertility clinics. During the past three years, she has served as a Laboratory Manager of Therapie Fertility and played a pivotal role in the establishment of its clinic in Dublin. In her recent role as Laboratory Manager, she led the establishment of Therapie Fertility Laboratory, the first MyGreen Lab certified IVF facility, emphasizing sustainability and also technological innovation for optimized efficiency and improved patient outcomes.
Dr Loscher is committed to enhancing the working environment for embryologists, ensuring that they are empowered to deliver affordable and accessible IVF care to couples in need.
Ian Muusha
AIan Muusha is a seasoned professional in the field of reproductive medicine, with a passion for innovation and sustainability. He has a background in embryology, having previously worked as an embryologist for one of the UK’s largest IVF treatment providers, and logistics, having worked for the world’s largest e-commerce corporations. Ian founded G-Fert Ltd with the vision of revolutionising the transportation of gametes and embryos, as well as biopsied cells and consumables for IVF Clinics, corporations and institutions. Committed to eco-conscious practices, Ian brings a unique blend of expertise in both the scientific and logistical aspects of reproductive healthcare. Through G-Fert, Ian aims to offer safe, efficient, and sustainable solutions to clinics and organisations worldwide.
Matt Guy
Matthew Guy, currently serving as a Consultant in the Service Department at CooperSurgical, brings a wealth of experience from his time in the operating theatres at Spital Davos, specifically within Gynaecology & Urology as well as working in service operations at CooperSurgical. Matthew's academic background in chemical engineering and biotechnology has laid a solid foundation for his work, culminating in the founding of FertiSene Analytics during his tenure at the University of Aalborg. This pioneering project employs predictive maintenance through IoT, data monitoring and machine learning analysis to anticipate equipment failures in IVF labs, marking a significant advancement in the field. Matthew's expertise in integrating engineering principles with reproductive health technology has made him a sought-after figure in the IVF community, where his work continues to influence best practices and innovative approaches to care.
Dr. Neelke De Munck
Neelke studied Biomedical Science and Molecular Biotechnology at the University of Ghent. Starting her career in pathology, she quickly made the switch to infertility in 2008. Initially starting a PhD on oocyte vitrification, she became a clinical embryologist in 2010 at Brussels IVF, Belgium. In 2018, she moved to Abu Dhabi to become the Scientific Director at ART Fertility Clinics. In 2021, she returned to Brussels IVF to become the head of the lab.
Dr Michael G. Collins
Michael has 25 years of reproductive health experience spanning medical affairs, clinical development, drug discovery, scientific communication, regulatory affairs, and managed markets and business development. He has also played an important role in the expansion of the reproductive health business units at both Ferring Pharmaceuticals and Organon Inc.
While at Ferring, Michael held various positions, including Director of Scientific Training, before moving to Director of Strategic Accounts, a role in which he built and led a strategic account team in the successful execution of several high-profile programs and projects.
Michael holds a Master of Science and a Doctor of Philosophy in Reproductive Physiology from Louisiana State University and has published work on many different levels within scientific literature. He is a member of ASRM, The Endocrine Society, and the Society for the Study of Reproduction, where he was appointed to the Annual Meeting Industry Relations Committee in 2013.
He is Managing Director, Scientific Affairs at TMRW Life Sciences.
At TMRW, Michael spearheads the development, design, and execution of laboratory studies, and then helps to communicate the findings. As a non-clinical scientist, his primary goal is to continue to scientifically define the TMRW platform. He’s passionate about bringing the advancements of cryogenic technology to reproduction.